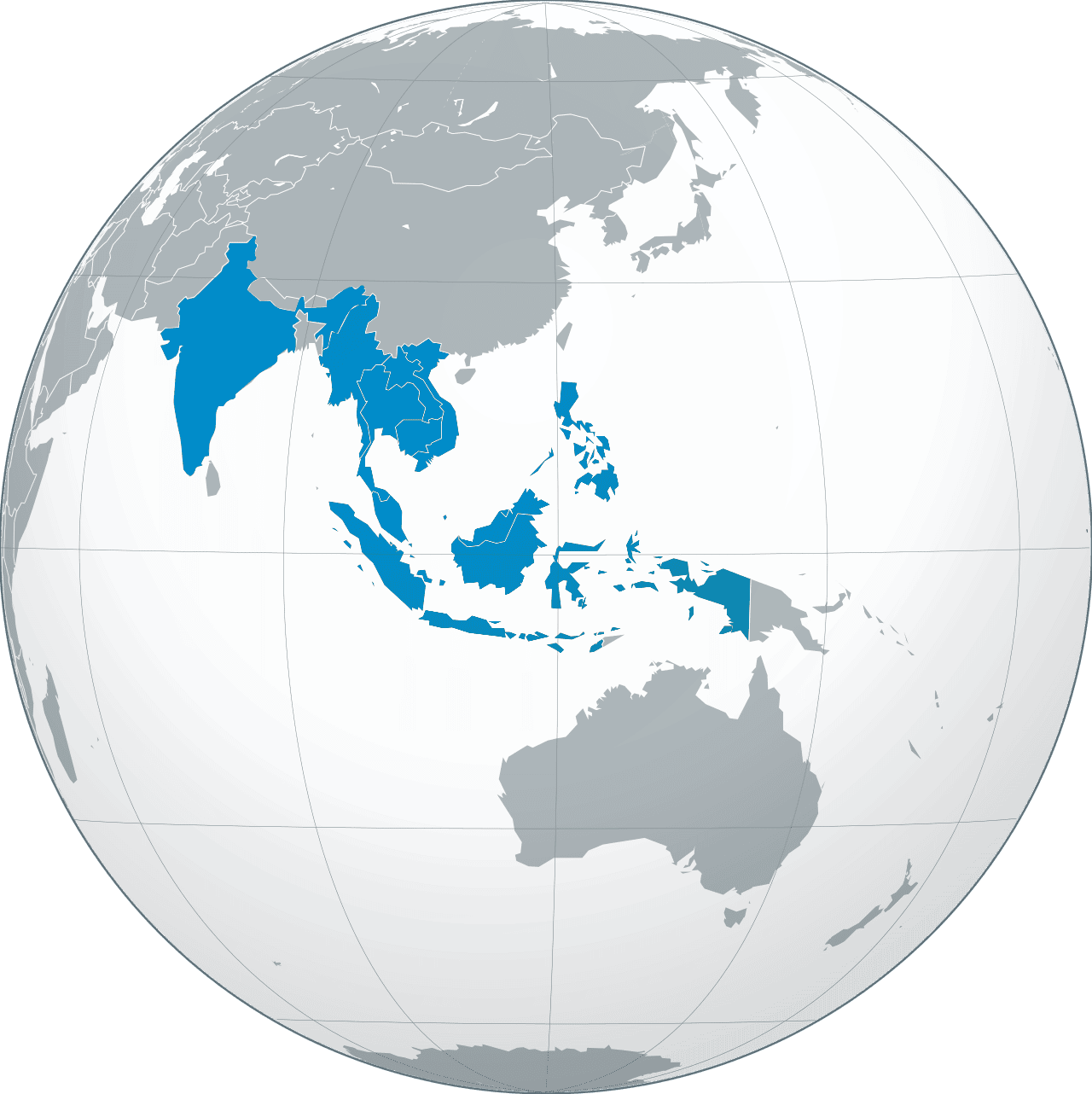
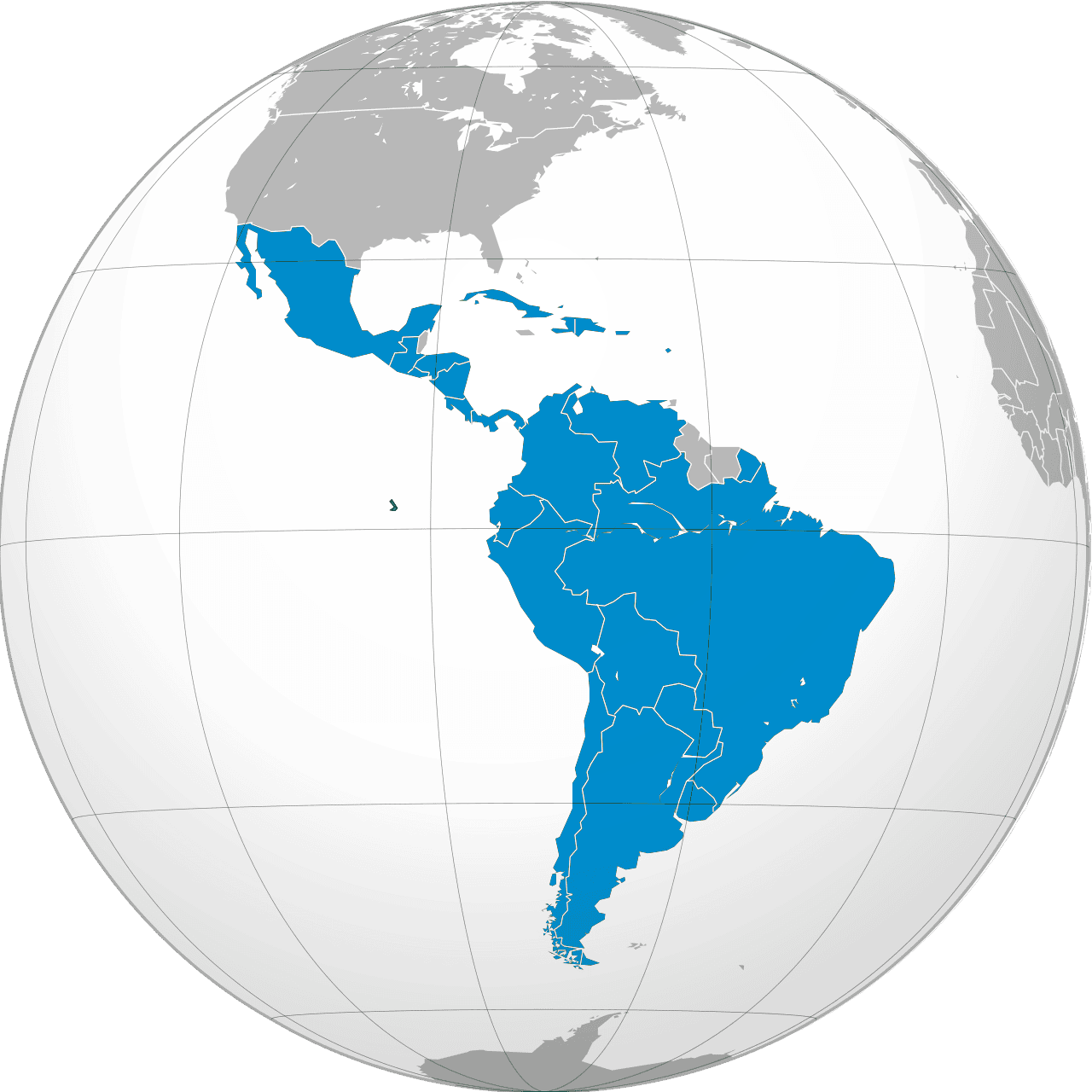
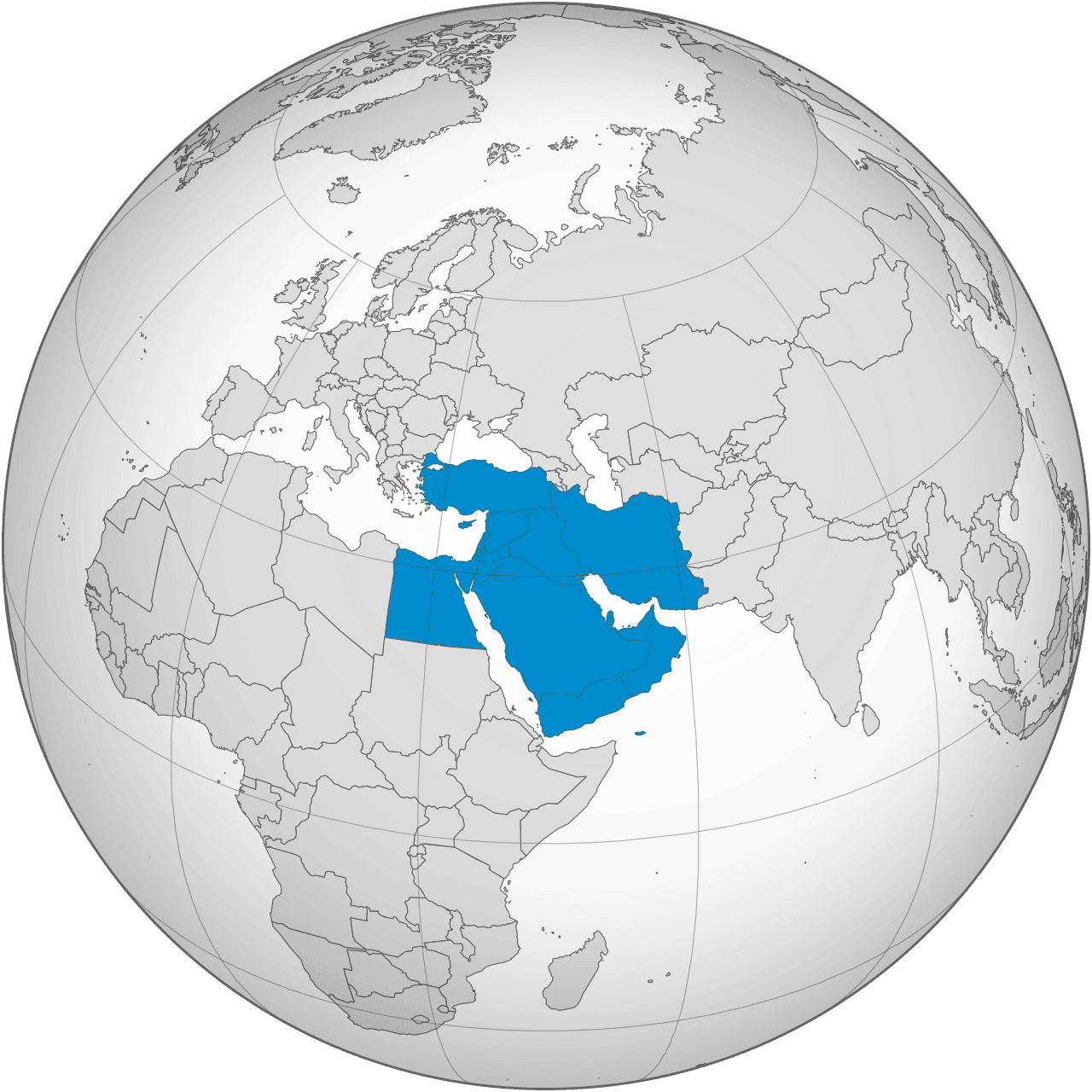
We operate in over 30 countries through subsidiaries, representative offices, partnerships, co-marketing initiatives, and a distribution-led model, ensuring direct market access and effective service in key geographies. By leveraging local expertise and collaborative networks, we deliver tailored solutions, expand our reach, and strengthen our position in priority markets. This adaptable approach fosters growth, builds lasting partnerships, and ensures sustainable success, reflecting our commitment to excellence and innovation in a competitive global landscape.